Background
The long-term survival of AML, especially of older AML patients, under the established therapies remains poor. A recent breakthrough therapy of selective BCL-2 inhibitor venetoclax with hypomethylating agents (HMA) azacitidine or decitabine is approved to treat older and unfit patients with AML (DiNardo et.al. EHA 2020). This combination induces responses in > 65% of older AML patients, but the responses in relapsed/refractory AML patients are sub-optimal. Upregulation of programmed death-1 (PD-1) in T-cells is associated with AML immune-suppression, and combination of anti-PD-1 with azacitidine has activity in relapsed/refractory AML (Daver et. al. Cancer Discovery 2019). Recent pre-clinical findings indicate that venetoclax preserved T-cells immunity by sparing central memory T-cells and enhanced activity of anti-PD-1 antibodies in immune-competent mouse models in vivo (Mathew et.al. Blood 2018). In this study, we tested the hypothesis that PD-1 inhibition facilitates the depth and duration of response to venetoclax and HMA combination by reactivating T-cells mediated immunity against AML.
Results
We collected peripheral blood (PB) samples from trial patients before and after receiving the treatment of decitabine and venetoclax (Dec_Ven) (NCT03404193) and examined the percentage of AML blasts and T-cells by multi-parameter flow cytometry. The combination of Dec_Ven effectively reduced PB CD33+/CD34+cells from 46 ± 15% at baseline (BL) to 27 ± 16% at 2.3 days (Day 1 - 3) (time point 1, or T1), and 15 ± 13% at 5.5 days (Day 3 - 8) (T2), and increased CD3+ T-cells: 28 ± 10% (BL), 54 ± 13% (T1) and 59 ± 19 % (T2), (BL vs.T1, CD33/CD34+, p = 0.001; CD3+, p = 0.02, n = 6). The reduction of CD33+/CD34+ positively correlated with the depletion of circulating blasts (R = 0.64, p = 0.0001).
Further analyzing the T-cells subsets among CD4helper cells and CD8cytotoxic cells, we found that Dec_Ven therapy promoted an activated T-cells phenotype by upregulating CD69 and PD-1 in both CD4and CD8cells in early time point samples, and led to T-cells exhaustion, as indicated by persistent expression of PD-1 at later time-point when CD69 was undetectable (PD-1 in CD3+CD4+ cells: 13 ± 2%, n = 6 (BL), 15 ± 3%, n = 6 (T1) and 22 ± 7%, n = 5 (T2); PD-1 in CD3+CD8+ cells: 14 ± 3%, n = 6 (BL), 27 ± 5%, n = 6, (T1), 29 ± 9%, n =5 (T2), BL vs. T1, p = 0.05). The persistent expression of PD-1 is a hallmark of T-cells exhaustion and immunosuppression (Jia et.al. Blood Cancer J 2018). Mass cytometry CyTOF immune-profiling of various compartments of CD4and CD8cells on PB uncovered that Dec_Ven reduced naïve T-cells (CD45RA+CCR7+) but spared or even increased frequencies of the central (CD45RA-CCR7+) and effector memory (CD45RA-CCR7-) CD4 or CD8 cells.
In parallel, we measured the serum cytokines levels, and found that treatment of Dec_Ven increased IL2, IL6 and INFg secretion in PB. IL2 and IL6 are two cytokines that regulate the proliferation and differentiation of effector memory T-cells; and INFg is a critical mediator of treatment-induced immune response.
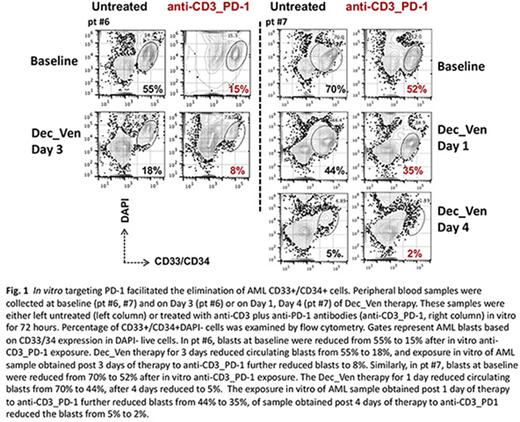
To further test if targeting PD-1 induced anti-leukemia effect and amplified the effect of Dec_Ven, we treated leukemia cells collected before and after Dec_Ven therapy with anti-CD3 and anti-PD-1 antibodies in vitro. Treatment reduced CD33+/CD34+ cells in 2 of 3 pre-treatment samples (untreated vs. anti-CD3_PD-1: 55% vs. 15%, pt #6; 70% vs. 52%, pt #7) and synergistically reduced/eliminated the residual CD33+/CD34+ cells in AML samples obtained after Dec_Ven therapy (untreated vs. anti-CD3_PD-1: 84% vs. 66% Day 3, pt #3; 18% vs. 8% Day 3, pt #6; 44% vs. 35% Day 1, 5% vs. 2% Day 4, pt #7) (Fig.1).
Conclusions
Our data indicate that Dec_Ven therapy induces PD-1 expression on T-cells but selectively preserves potentially important anti-tumor T-cells immune subsets. The "triple" combination therapy of HMA with BCL-2 and PD-1 antagonists may facilitate anti-leukemia responses by enhancing T-cells function. Clinical trial to test this hypothesis is ongoing in relapsed/refractory AML patients (NCT02397720).
DiNardo:Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; Agios: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Novartis: Consultancy; MedImmune: Honoraria; Takeda: Honoraria; Syros: Honoraria; ImmuneOnc: Honoraria; Notable Labs: Membership on an entity's Board of Directors or advisory committees. Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Konopleva:Agios: Research Funding; Ascentage: Research Funding; AstraZeneca: Research Funding; Sanofi: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Eli Lilly: Research Funding; Calithera: Research Funding; Kisoji: Consultancy; Stemline Therapeutics: Consultancy, Research Funding; Amgen: Consultancy; F. Hoffmann La-Roche: Consultancy, Research Funding; Cellectis: Research Funding; Ablynx: Research Funding; AbbVie: Consultancy, Research Funding; Forty-Seven: Consultancy, Research Funding; Rafael Pharmaceutical: Research Funding; Genentech: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal